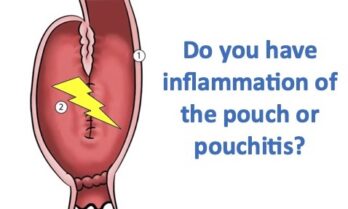
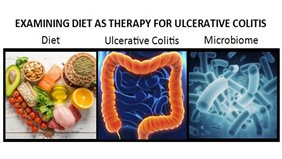
Current research
CCA supports research through encouraging members to participate in research, advertised in our eNews, member magazine and here on our website.
Research ranges from clinical trials in drug therapy and dietary investigations, to population surveys to discover information and cause, prevention, treatment and quality of life.